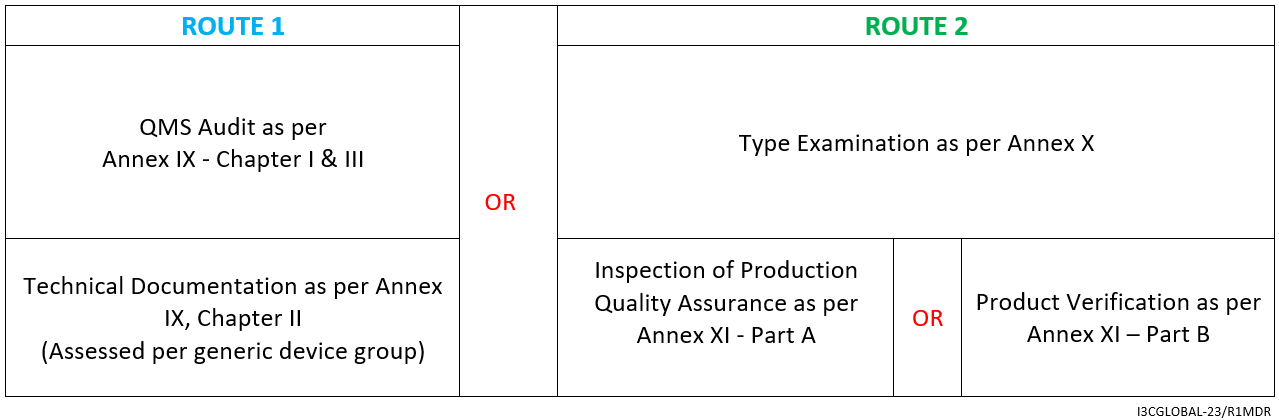
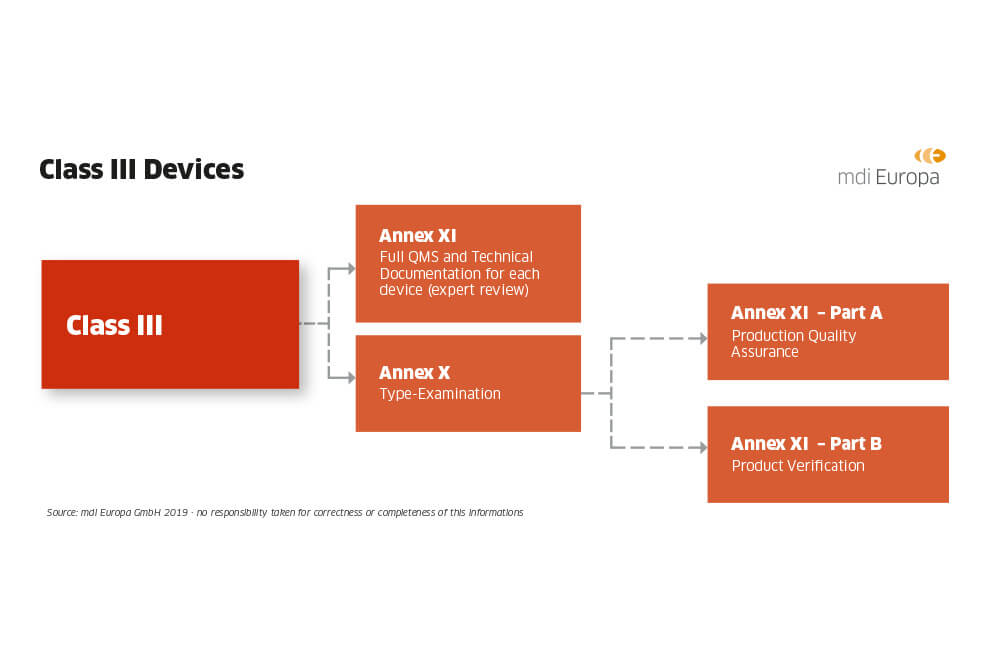
DEVICE REGULATIONS - The New Medical Device Regulation & the Applicability of Article 117 to Medicinal Products
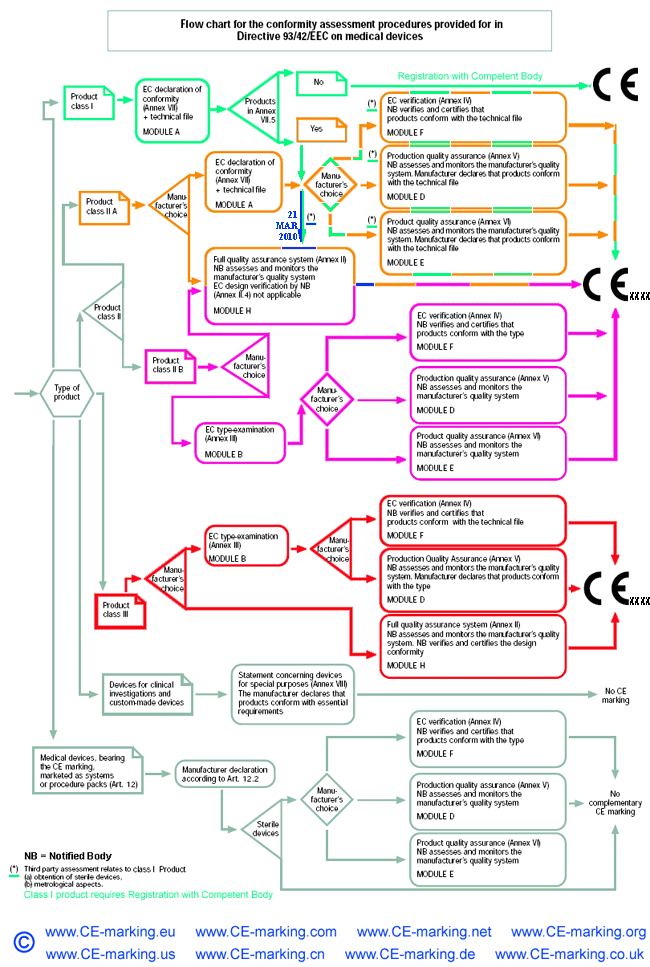
Guide on Class I (Is/Im) MDD- Medical Devices CE marking (mark) & European ( EU) Authorized Representative service
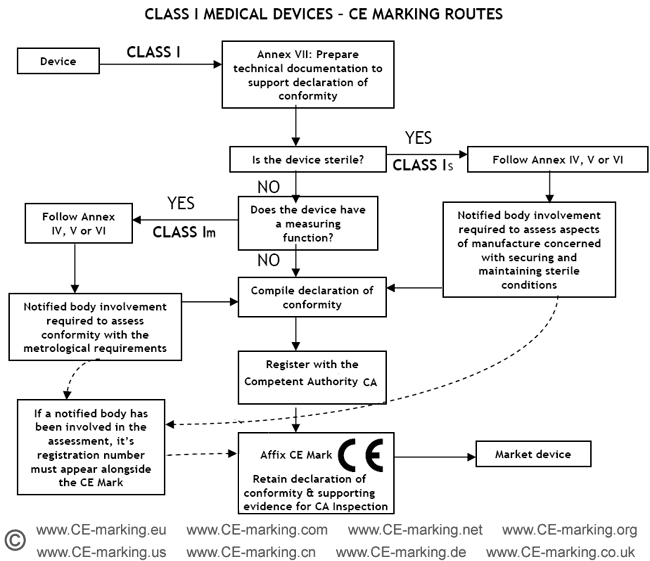
Guide on Class I (Is/Im) MDD- Medical Devices CE marking (mark) & European ( EU) Authorized Representative service