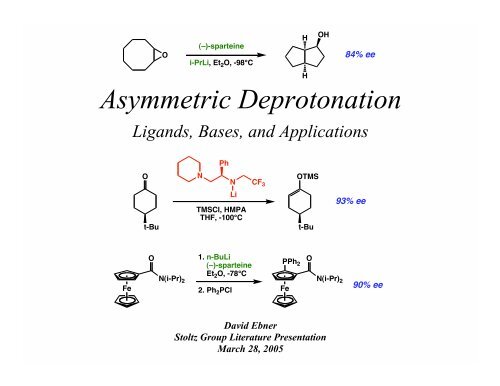
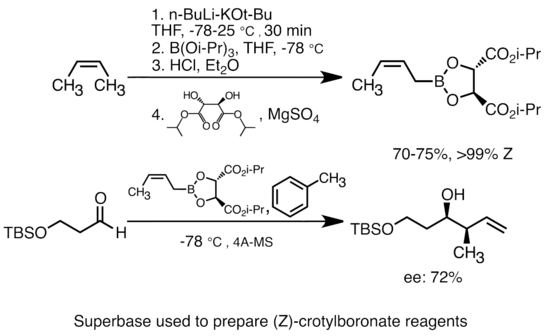
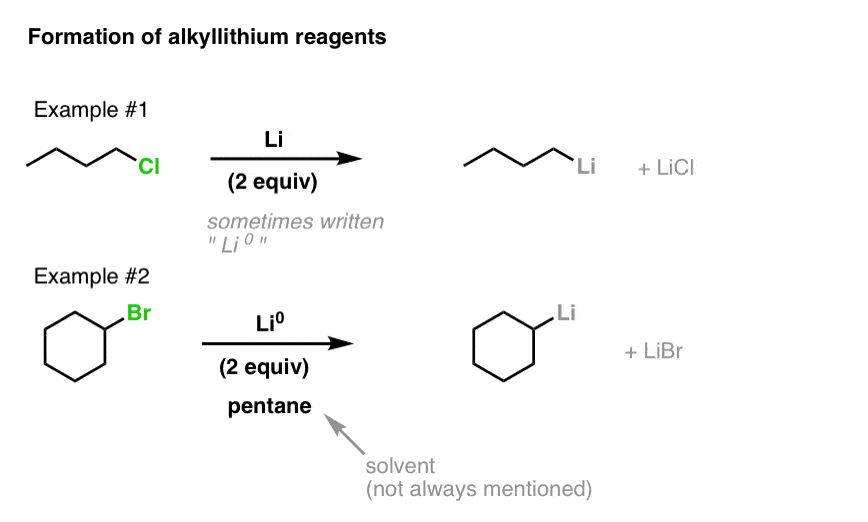
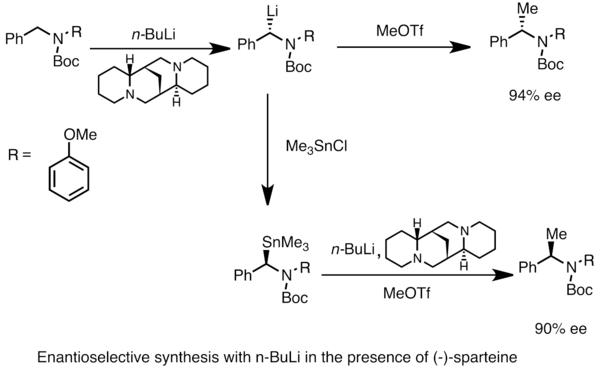
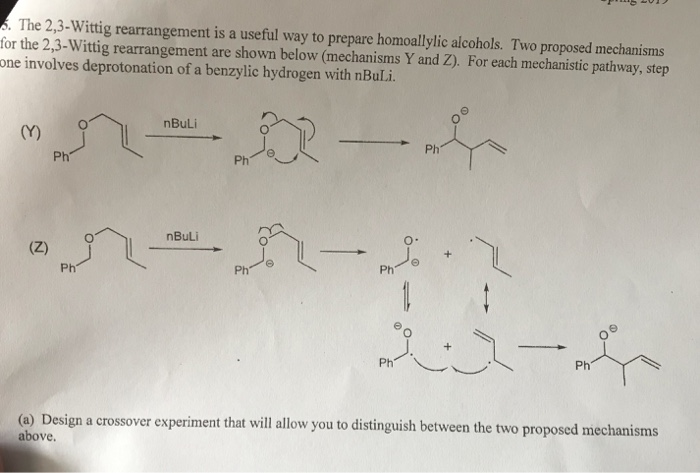
WO2009056487A1 - Methods for stabilizing lithiated halogen-substituted aromatic compounds - Google Patents
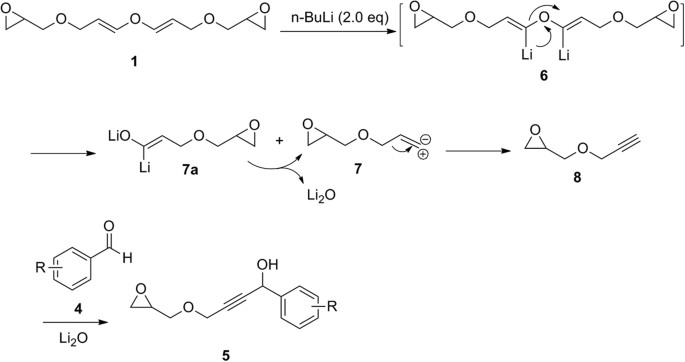
Unprecedented reactions: from epichlorohydrin to epoxyglycidyl substituted divinyl ether and its conversion into epoxyglycidyl propargyl ether | Scientific Reports

Scheme 1 o-Phenylthiostyrene oxide (R)-4 was deprotonated at 157 K with... | Download Scientific Diagram

n‐Butyllithium‐mediated reactions of 1‐(2‐azidoarylmethyl)‐ 1H‐benzotriazoles with alkyl halides - Kim - 2010 - Journal of Heterocyclic Chemistry - Wiley Online Library
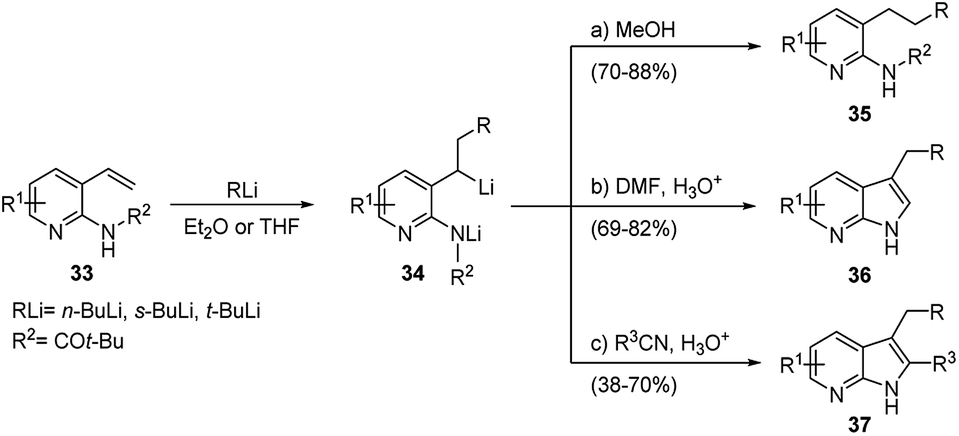
Regio- and stereoselective intermolecular carbolithiation reactions - RSC Advances (RSC Publishing) DOI:10.1039/D0RA06101H

Mechanism of the Deprotonation Reaction of Alkyl Benzyl Ethers with n‐ Butyllithium - Raposo - 2013 - Chemistry – A European Journal - Wiley Online Library

organic chemistry - nBuLi and tBuLi can take part in halogen metal exchange OR deprotonate. Is there any way to predict which it favours? - Chemistry Stack Exchange
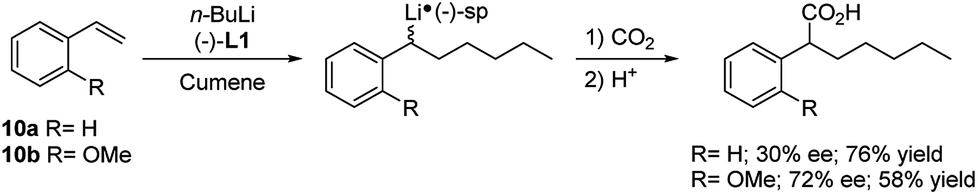
Regio- and stereoselective intermolecular carbolithiation reactions - RSC Advances (RSC Publishing) DOI:10.1039/D0RA06101H
![Selective deprotonation of tetra[3,4]thienylene in the presence of n-BuLi - Organic Chemistry Frontiers (RSC Publishing) Selective deprotonation of tetra[3,4]thienylene in the presence of n-BuLi - Organic Chemistry Frontiers (RSC Publishing)](https://pubs.rsc.org/en/Content/Image/GA/C6QO00754F)