A chemoselective deprotection of trimethylsilyl acetylenes catalyzed by silver salts - ScienceDirect
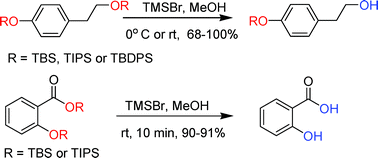
The chemoselective and efficient deprotection of silyl ethers using trimethylsilyl bromide - Organic & Biomolecular Chemistry (RSC Publishing)

Boric acid as cost-effective and recyclable catalyst for trimethylsilyl protection and deprotection of alcohols and phenols

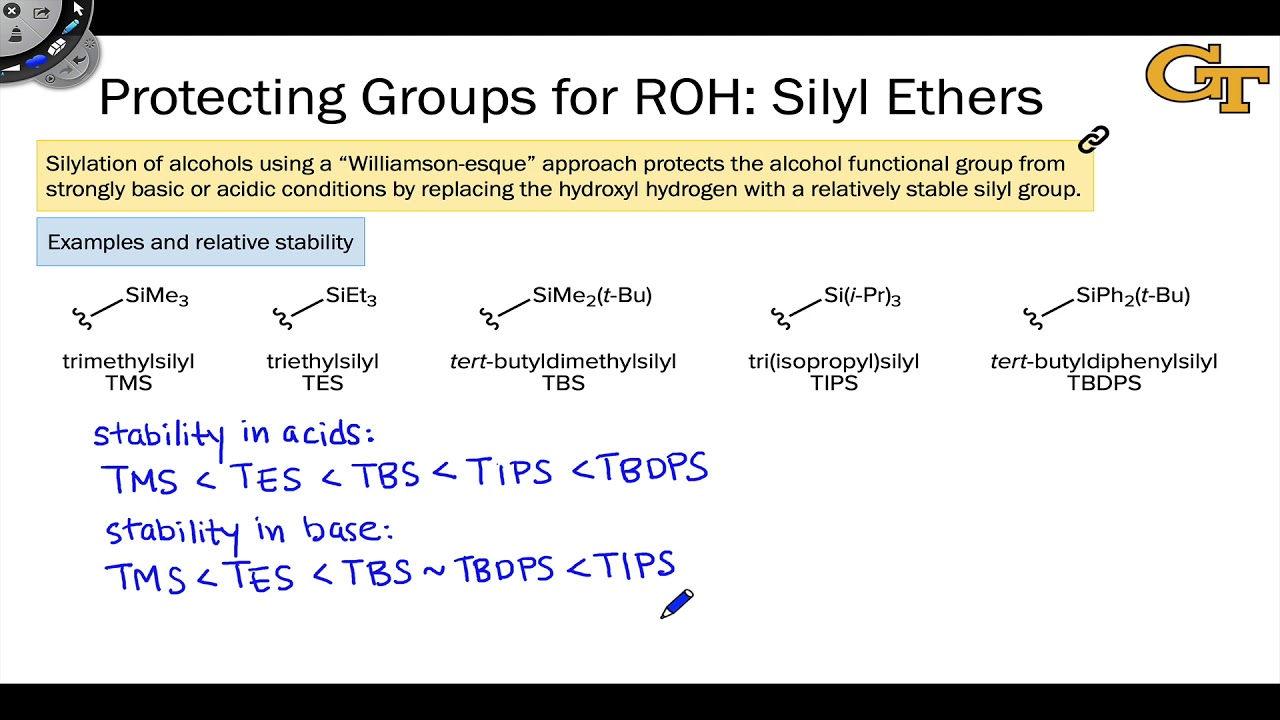
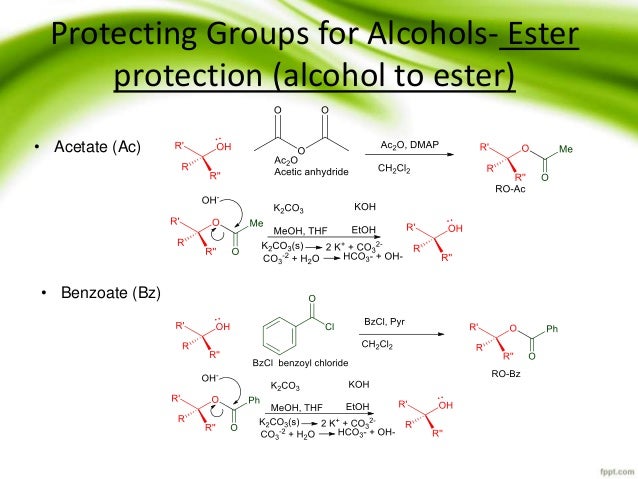
Protection and deprotection of alcohols by a common silyl ether - trimethylsilyl (TMS) | Chemistry lessons, Organic chemistry, Chemistry

A chemoselective deprotection of trimethylsilyl acetylenes catalyzed by silver salts - ScienceDirect

Reaction mechanism of the methylation of a carboxylic acid R-COOH with... | Download Scientific Diagram
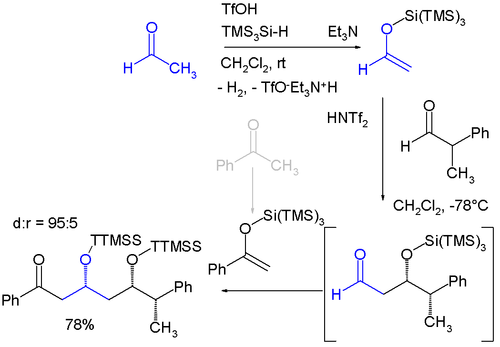
Protecting and Leaving Functions of Trimethylsilyl Groups in Trimethylsilylated Silicates for the Synthesis of Alkoxysiloxane Ol
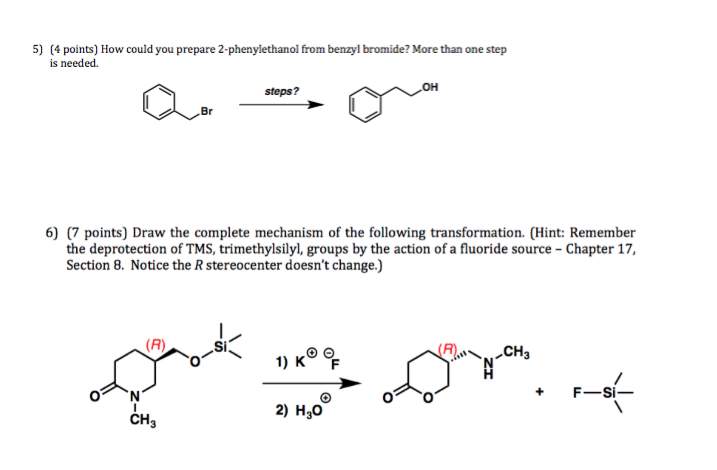
An Efficient Method for Selective Deprotection of Trimethylsilyl Ethers and Tetrahydropyranyl Ethers under Solvent-free Conditio
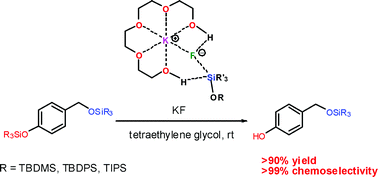
A mild and efficient method for the selective deprotection of silyl ethers using KF in the presence of tetraethylene glycol - Organic & Biomolecular Chemistry (RSC Publishing)

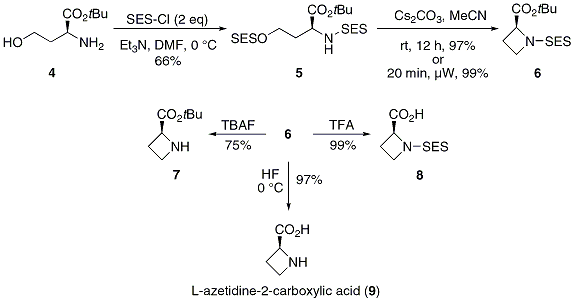
Is there a method to eliminate the trimethylsilyl group from carboxyl or amine ends of a peptide chain after HMDS mediated NCA polymerization?

A Novel Strategy for Selective <i>O</i>-Methylation of Glycerol in Subcritical Methanol. - Abstract - Europe PMC